New Laser for 3P Microscopy: The tunable White Dwarf WD-1300-1700-pro
We are thrilled to announce the release of our newest White Dwarf laser system specifically engineered for three-photon (3P) microscopy. This cutting-edge system combines the highest peak power available for 3-photon imaging, along with tunability covering the 1300 nm and 1700 nm regions.
- The White Dwarf WD-1300-1700-pro
|
|
|
1. Introduction
High-resolution deep-tissue imaging has emerged as a major focus of research across many disciplines. The ability to visualize biological structures in-vivo and in a non-invasive manner is crucial for understanding the complex functions of living cells, tissues, and organs under real world conditions. However, conventional imaging techniques face significant challenges when imaging deep into tissues, primarily due to the strong scattering and absorption of light in the upper tissue layers, limiting not only depth but also resolution [1].
Three-photon fluorescence microscopy (3P microscopy) was developed to image beyond the limits of two-photon microscopy and has achieved unprecedented in-vivo imaging depths [2]. 3p microscopy uses ultrashort femtosecond lasers with near-infrared central wavelengths centered at 1300 nm and 1700 nm, targeting existing green and red fluorescent markers, respectively. These wavelengths experience reduced scattering and absorption within brain and other organic tissues. Furthermore, the highly nonlinear three-photon absorption process further enhances the signal-to-noise ratio by minimizing out-of-focus fluorescence. In particular, because of its highly nonlinear nature, three-photon absorption takes place exclusively at the laser’s focal point, where the light intensity is at its maximum.
The WD-1300-1700-pro for 3p microscopy offers the following key advantages:
- High peak power and pulse compression quality: With the highest peak power available for three-photon microscopy, the WD-1300-1700-pro allows for deeper imaging and with higher contrast in scattering tissues than ever before.
- Dual Wavelength Tunability at 1300 nm & 1700 nm: Effortlessly tuning and switching between 1300 nm (tunable from 1250 nm – 1350 nm) and 1700 nm (tunable from 1650 nm – 1750 nm) with our stand-alone control software.
- Field-Proven Design: Trusted by more than 30 neuroscience labs worldwide, with a growing list of publications in top-tier journals demonstrating its capability in applications like Adaptive Optics (AO) and miniaturized, head-mounted microscopy.
- Reliable Performance: Experience long-term stability with our robust, one-box solution, simplifying your setup.
2. System Overview
The White Dwarf WD-1300-1700-Pro is a robust, turnkey, one box laser system (Fig. 2) with integrated industrial pump laser and tunable high power optical chirped pulse parametric amplifier (OPCPA). In addition, the system includes built-in compressor and accurate dispersion compensation, enabling the highest pulse compression quality and, thus, contrast, in the targeted tissue depth.

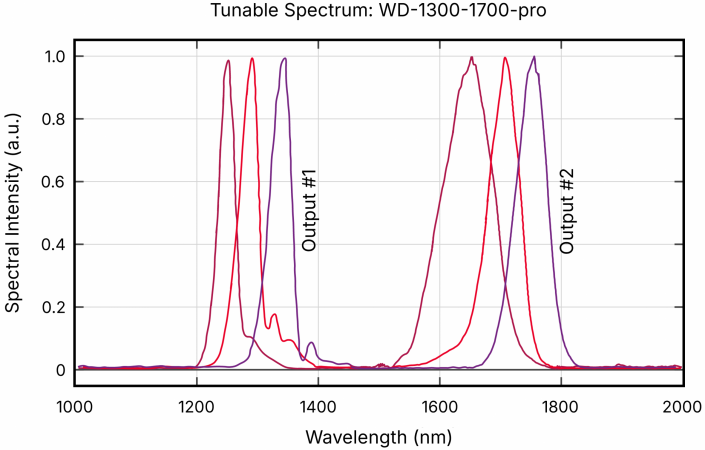
The White Dwarf WD-1300-1700-Pro has two different switchable outputs: Output 1, centered at 1300 nm and tunable from 1250-1350 nm; and Output 2, centered at 1700 nm and tunable from 1650-1740 nm (Fig. 3). A third output, centered at 1035 nm for optogenetics or 2-photon imaging, is also optionally available. Both 3P outputs deliver femtosecond laser pulses.
The pulse duration is <50 fs over the entire tunable range for Output 1 (Fig. 4), and <65 fs for Output 2 (Fig. 5). Both outputs exhibit remarkable long-term power stability (Fig. 6).
|
|
|
3. Customer Examples
For more than a decade, our White Dwarf platform has been developed and continuously improved in close collaboration with leading research laboratories at some of the world’s most prestigious universities, including:
- Rockefeller University (USA)
- Columbia University (USA)
- Max Planck Caesar Bonn (DE)
- Max Planck Florida Institute for Neuroscience (USA)
- Sorbonne University (FRA)
- EMBL Heidelberg (DE)
- Yale University (USA)
- Stanford University (USA)
- Johns Hopkins University (USA)
- University College London (GB)
White Dwarf systems have empowered researchers at these and many other institutions in their pursuit to push the boundaries of multiphoton microscopy. The next customer success stories highlight how the White Dwarf unlocks new insights into the complexities of the brain:
3.1. High-speed volumetric brain imaging combined with Three Photon Microscopy
| The Vaziri Lab from the Rockefeller University developed an advanced in-vivo microscopy technique which is designed for high-speed, volumetric imaging of deep neural activity[3]. They employ simultaneous multiplexed two-photon (2P) and single line three-photon (3P) microscopy using GFP as a fluorophore. With this approach, they are able to image a substantial volume encompassing an entire cortical column (volume of approximately 1 mm³) at a rapid frame rate of up to 17 Hz. | 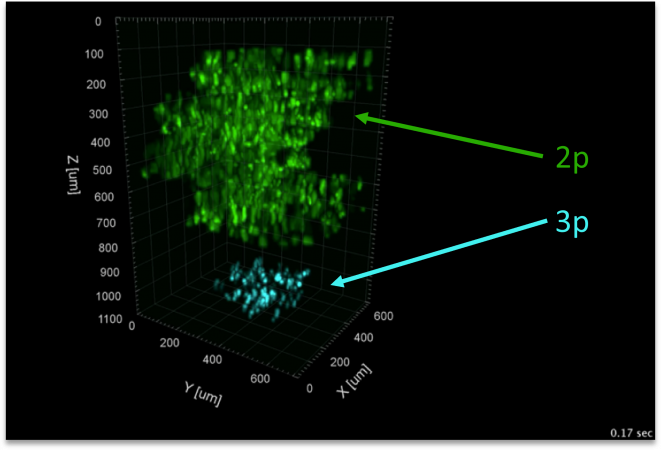 |
Advantages of the White Dwarf that supported this research
| High pulse energies: | High repetition rates: |
|
|
| The Prevedel lab from EMBL Heidelberg presented a new, custom-built high-performance imaging system that achieves near-diffraction-limited resolution up to an in-vivo imaging depth of over 1.4 mm by combining three-photon (3P) microscopy (using GFP) with a sophisticated adaptive optics (AO) setup [4]. The AO component is specifically designed as an indirect, modal-based approach, which effectively corrects for optical aberrations in deep, scattering tissue to maintain high imaging resolution. | 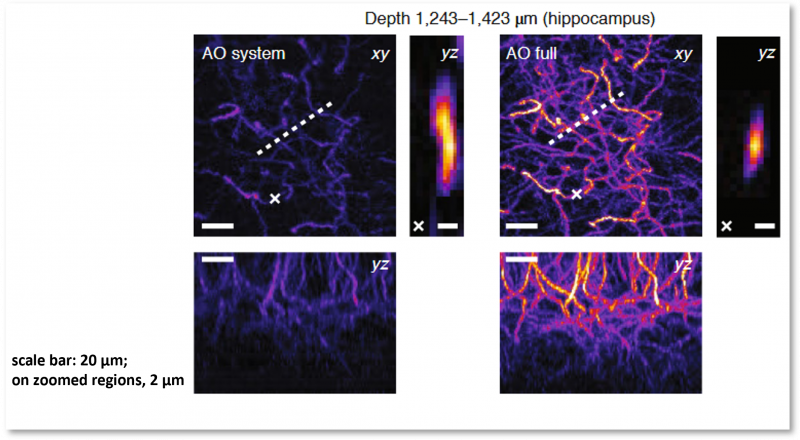 |
Advantages of the White Dwarf that supported this research
| Custom dispersion compensation: | High pulse energies: | High stability: |
|
|
|
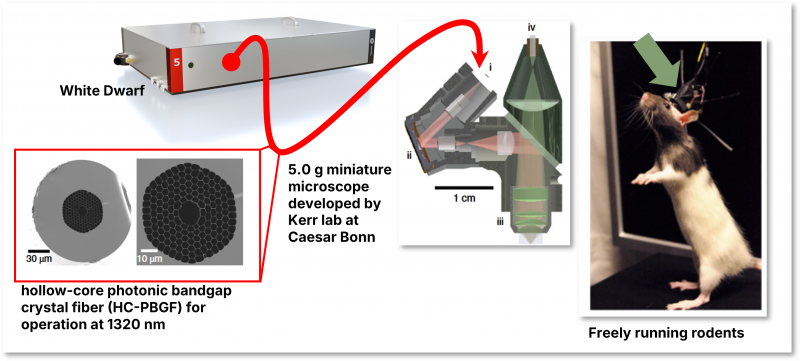
Advantages of the White Dwarf that supported this research
| Custom dispersion compensation: | High pointing stability: |
|
|
| The Kerr lab from Max Planck Institute Caesar in Bonn improved their developed head-mounted microscope system further to perform two-photon (2P) and three-photon (3P) imaging simultaneously. Utilizing this dual-modality, the system enables imaging of neuronal activity across five vertically separated planes, allowing researchers to capture activity from cell populations across both superficial and deep cortical layers [7]. | 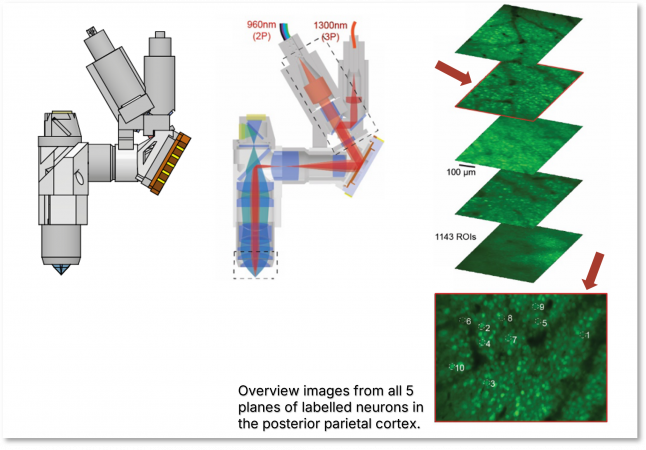 |
Advantages of the White Dwarf that supported this research
| Custom dispersion compensation: | Simultaneous dual channel operation: | High pointing stability: |
|
|
|
- Nature Collection: Microscopic Imaging in Deep Tissue. 09 April 2024. https://www.nature.com/collections/hgaebhdjfg
- Xiao, Y. et al. Three-photon excited fluorescence imaging in neuroscience: From principles to applications. Front Neurosci. 17, 1085682 (2023). https://doi.org/10.3389/fnins.2023.1085682
- Weisenburger, S. et al. Volumetric Ca2+ imaging in the mouse brain using hybrid multiplexed sculpted light microscopy. Cell 177, 1050–1066 (2019). https://doi.org/10.1016/j.cell.2019.03.011
- Streich, L. et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nature Methods, 18, pages 1253–1258 (2021). https://doi.org/10.1038/s41592-021-01257-6
- Klioutchnikov, A. et al. Three-photon head-mounted microscope for imaging deep cortical layers in freely moving rats. Nature Methods, 17, pages 509–513 (2020). https://doi.org/10.1038/s41592-020-0817-9
- Klioutchnikov, A. et al. A three-photon head-mounted microscope for imaging all layers of visual cortex in freely moving mice. Nature Methods 20, pages 610–616 (2023). https://doi.org/10.1038/s41592-022-01688-9
- (Preprint) Klioutchnikov, A. et al. Simultaneous 2- and 3-photon multiplane imaging across cortical layers in freely moving mice (2025). https://doi.org/10.1101/2025.08.01.668113