Scaling functional brain imaging with advanced high-power multi-photon microscopy
1. Advanced multi-photon imaging in neuroscience
In recent years, the advancements in neuroscience have been nothing short of revolutionary. Advanced two- and three-photon imaging methods, once the domain of pioneering labs, are becoming increasingly sophisticated and mainstream in research settings. In addition, core facilities start adapting two-photon (2P) and three-photon (3P) microscopy for providing imaging services to users. Robustness and reliability of the laser source are the main success factor.
With the refinement of multi-photon imaging methods, we are able to capture high-speed brain activity with greater resolution, dive deeper into the brain’s structures using 3P imaging, and achieve broader fields of view. Holographic techniques, though demanding in terms of peak power, open new routes in imaging, while fiber- coupled mini-scopes provide versatility in real-time, in-vivo visualization. Adaptive optics have taken center stage, delivering sub-cell resolution, and elucidating the nuances of neuron-axon interactions.
In this work, we spotlight how high-power, high-repetition-rate lasers are revolutionizing this domain, amplifying our understanding from individual neuronal dynamics to comprehensive ensemble interplay.
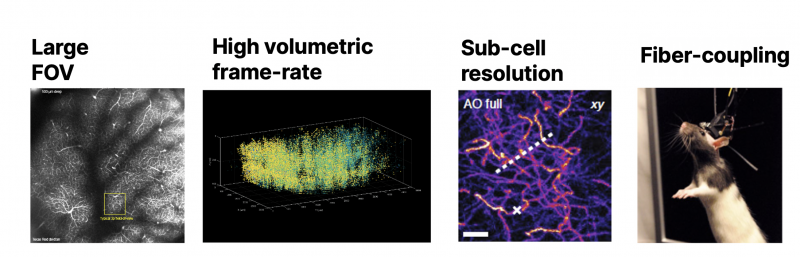
The motivation for these techniques in neuroscience is straightforward: We want to use light as a non-invasive tool to measure and control neuronal activity. We claim that in order to achieve meaningful results for biology at least one of the following criteria must to be fulfilled by the imaging method (Fig. 1):
- Imaging depth: In order to reach deeper layers in vivo without removing brain tissue, especially 2P and 3P techniques aim to reach depths well beyond 1 mm. This requires laser pulses with sub-100 fs pulse duration at specific wavelengths, for example 920 and 960 nm (2P), and 1300 and 1700 nm (3P) in green and red fluorescent genetic protein markers [1–3]
- Large field-of-view: New optical set-ups and objectives allow for large field-of-view (FOV) of a few mm2 in order to map all neurons of a larger brain area [4]
- Large volume: The combination of large FOV and imaging depth gives a large volume, allowing to image an unprecedented number of ensembles of neurons
- High volumetric frame rate: Various strategies for fast scanning techniques have been implemented, some of which enabling the in-vivo imaging of hundreds of thousands of neurons, allowing to record their activity in real-time. High laser peak power and repetition rate is required, resulting in average power levels above 5 W, and power stability of the laser source [1, 5]
- Sub-cell resolution: For resolving fine neuronal structures, such as axons, dendrites and synapses in deep tissue, advanced adaptive optics systems are being used to achieve diffraction-limited, sub-cell resolution. Due to the low throughput of these complex optical systems, high power lasers are required, and very accurate beam pointing stability is required [2]
- Fiber-coupling: Miniscopes for 2P and 3P imaging allow for 3-dimensional measurement of the real-time brain activity of a free-running animal. The laser system is connected to an ultra-compact, fiber optic-coupled microscope with the mouse. A key necessity of the laser source is stable, high-power output with very accurate beam-pointing stability in order to maintain the coupling to the optical fiber [3]
2. High-power femtosecond lasers for 2P and 3P imaging
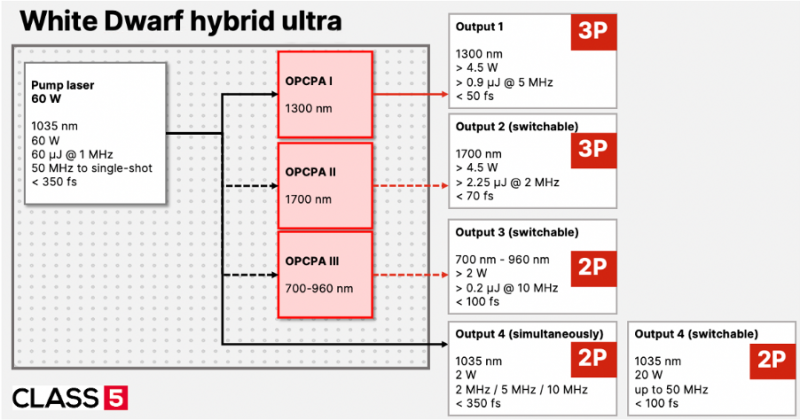
The high peak power required is often achieved through pulse energies of more than 1 μJ at pulse durations below 50 fs. At increasing repetition rate up to 5 MHz, the resulting average power before the microscope is 5 W. In addition, these parameters need to be achieved at specific wavelengths of 920, 960 (2P) and 1300 or 1700 nm (3P), which are not attainable by traditional laser systems. We use a combination of industrial high-power lasers and advanced nonlinear optical techniques to realize very powerful and robust laser systems optimized for bio-imaging. This includes optical-parametric chirped-pulse amplification (OPCPA, [6]) and white-light generation. Our OPCPA system is well scalable in average power and the high gain allows for very compact laser systems, and multiple OPCPA outputs for optogenetics, 2P and 3P imaging from a single platform (Fig. 2).
References
- Demas,J., Manley,J., Tejera,F., and others. High-speed,cortex-widevolumetricrecordingofneuroactivityatcellularresolu- tion using light beads microscopy, Nature Methods 18, 1103–1111 (2021)
- Streich, L., Boffi, J.C., Wang, L., and others. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat Methods 18, 1253–1258 (2021)
- Klioutchnikov, A., Wallace, D.J., Sawinski, J., Kerr, J., and others. A three-photon head-mounted microscope for imaging all layers of visual cortex in freely moving mice. Nat Methods (2022).
- Che-HangYuetal. The Cousa objective: alongworkingdistanceairobjectiveformultiphotonimaginginvivo, bioRxiv 2022.11.06.515343
- Kazemipour, A., Novak, O., Flickinger, D., and others. Kilohertz frame-rate two-photon tomography. Nat Methods 16, 778–786 (2019).
- Dubietis A, Matijosˇius A. Table-top optical parametric chirped pulse amplifiers: past and present. Opto-Electron Adv 6, 220046 (2023)


