Multiphoton microscopy
Application
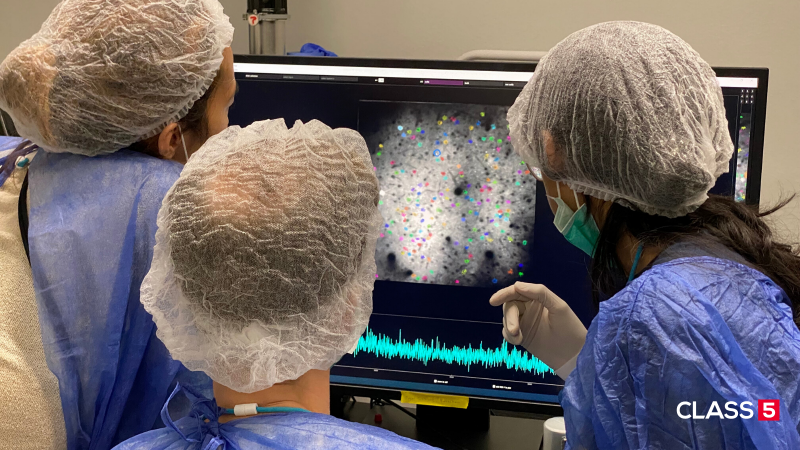
Fluorescence microscopy enables in vivo non-invasive imaging of cells. For example, neuronal activity within the brain can be observed in real-time. The most interesting brain regions, such as parts of the hippocampus in mice model systems, are below the maximum penetration depth of today’s two-photon microscopes that operate between 700 and 1040 nm wavelength. To overcome this limitation novel three-photon microscopy with near-infrared lasers at 1300 and 1700 nm is being implemented. The longer wavelengths are less diffracted in the brain tissue and the three-photon absorption improves the signal-to-noise ratio by suppressing out-of-focus fluorescence. Using our powerful White Dwarf product family allows recording a large volume of cells in real-time (i.e. at high frame-rate) and with sub-cell resolution.
Neuroscience
How do feelings arise? How does the sense of touch work? How are complex decisions made?
These are research questions for biologists and neuroscientists who aim for a better understanding of how the brain functions. The key technology for neuroscientists allowing to record brain activity in vivo is fluorescence microscopy.
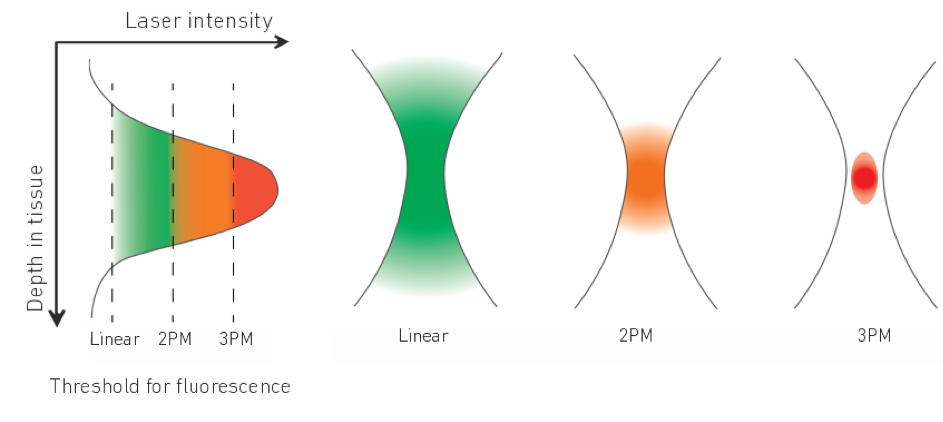
However, in conventional linear fluorescence microscopy already a considerable proportion of the laser intensity is scattered in upper tissue layers which prevents imaging deeper regions of the brain. Nowadays, two-photon fluorescence microscopy is the standard technique for in vivo brain imaging with single-cell resolution also deep inside the tissue. In two-photon microscopy, two photons of longer wavelengths (near-infrared 700-1040 nm) are employed which are absorbed simultaneously to excite the fluorophore. Less scattering of the longer wavelengths allows imaging down to 600 µm inside the tissue.
The most interesting brain regions, such as parts of the hippocampus in mice model systems, are even below the maximum penetration depth of today’s two-photon microscopes.
3-photon microscopy
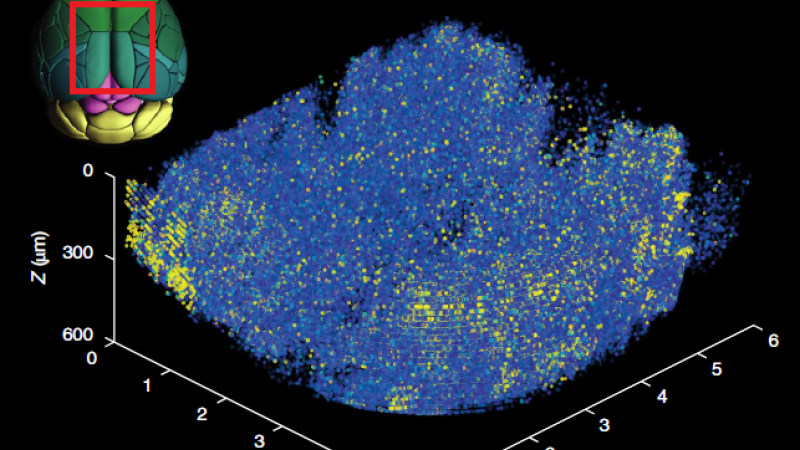
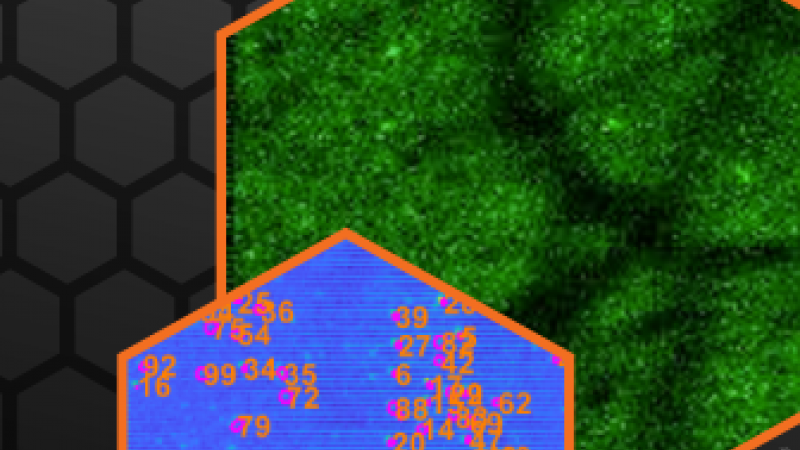
To overcome this depth limitation novel three-photon microscopy (3P) with near-infrared lasers having ultrashort pulses (50-70 fs) at 1300 and 1700 nm wavelengths is being implemented. The even longer wavelengths are even less diffracted in the brain tissue and the three-photon-absorption improves further the signal-to-noise ratio by suppressing out-of-focus fluorescence. Specifically, due to the non-linear effect, three-photon absorption only occurs in the laser focus where the laser intensity is highest. Hence, areas below 1 mm inside the brain can be imaged without being obscured by fluorescence before the laser focus.

Recently, very advanced, new strategies for beam and pulse shaping in three-photon microscopes have been developed. Those allow imaging of even deeper brain regions with near-diffraction-limited resolution or the scanning of very large volumes on the mm3 scale and almost in real-time. Such applications benefit from the high performance and stability of the near-infrared laser system. The peak power in the volume pixel (voxel) to be imaged ultimately defines the three-photon absorption and hence fluorescence signal response. This means for imaging even deeper, or at better resolution, high pulse energies of a few μJ and short pulses below 50 fs at 1300 nm and 70 fs at 1700 nm are needed. And for gaining speed at such comparably high pulse energies, repetition rates in the MHz range are required – the ideal conditions for our powerful White Dwarf 3P.

In addition, core facilities start adapting three-photon microscopy for providing imaging services to users. Robustness and reliability of the laser source are the main success factor. High up-time with easy serviceability is required. That’s why we decided to implement a robust industrial laser as pump source for the White Dwarf 3P.
Related Products
Publications
-
September 30, 2021Streich, L., Boffi, J.C., Wang, L. et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat Methods 18, 1253–1258 (2021).
-
November 28, 2022Klioutchnikov, A., Wallace, D.J., Sawinski, J. et al., A three-photon head-mounted microscope for imaging all layers of visual cortex in freely moving mice, Nat Methods (2022).
-
August 30, 2021Demas, J., Manley, J., Tejera, F. et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy. Nat Methods (2021).
-
May 2, 2019Weisenburger et al., Volumetric Ca2+ Imaging in the Mouse Brain Using Hybrid Multiplexed Sculpted Light Microscopy, Cell (2019)